Cochlear & Implantable Hearing Devices
Implantable Hearing Devices
Implantable hearing aids are available for those who cannot benefit from conventional “off the shelf” or custom made hearing aids. All implantable hearing devices are comprised of an internal component which is surgically implanted by an ear surgeon and an external processor worn over the skull which is fitted, programmed and maintained by an audiologist. They all operate on batteries. To date, there is still no commercially available device totally implanted and invisible. Interestingly, conventional hearing aids are still far less visible than any implant. There are different implantable options to address a variety of hearing and ear disorders.
Cochlear Implants
Implantable technology is only indicated in those who cannot wear a conventional hearing aid to facilitate their hearing habilitation process.
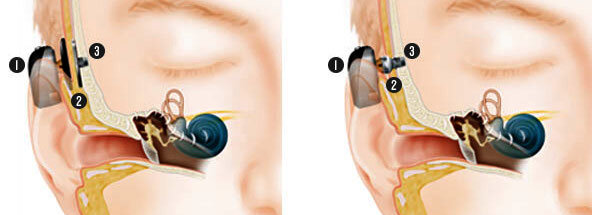
As technology advances, more adults with acquired and congenital (born with) hearing losses are taking advantage of cochlear implant technology, as there is no age restriction. One can be as young as 3 months or as old as 100 to benefit from a hearing implant. All models have an internal part which is surgically implanted and an external part much like a conventional hearing aid. The external part is referred to as “speech processor” or “sound processor”.
Cochlear implants electrically stimulate the hearing nerve directly via electrodes implanted in the cochlea and connected to the external processor via a magnet placed under the skin on the skull behind the ear. It is suitable for those with more severe sensorineural hearing impairments with an intact auditory nerve.
The sound processor which is attached to the internal implant via a magnet is available in 2 different styles: behind-the-ear with the main part sitting on the pinna and connected via a cable to the coil placed on the skull or one single unit placed on the skull without touching the ear.
Dr McNeill with Cochlear Implant patient
Electro-Acoustic implants
Electro-Acoustic stimulation (EAS) or Hybrid is a combination of a cochlear implant and a conventional hearing aid in the same device. The indication is for those with reasonably good low-frequency hearing and no hearing in the high frequencies. The cochlear implant portion of the device provides the high frequencies electrically and the low frequencies are acoustically supplied by a hearing aid.
Bone-conduction implants
Bone-anchored hearing aids provide hearing via bone conduction stimulating the cochlea through vibration of the skull. They are commonly referred to as BAHA although this acronym is now trademarked by Cochlear Ltd.
There are two different styles using different modes of bone conduction stimulation. One uses an abutment screwed into the mastoid bone behind the ear and the other uses a magnet placed under the skin. The implant is connected to an external sound processor either attached to the abutment as per the Cochlear BAHA Connect and the Oticon Medical Ponto, or secured by a magnet like the Medel Bonebridge and the Cochlear BAHA Attract.
These implants are suitable for those who have a hearing loss due to external or middle ear disorders such as malformation of the external ear, chronic ear discharge and allergic reactions to plastic which prevent wearing conventional hearing aids.
Another indication is for those with one normal hearing ear and are totally deaf in the other ear who do not want to wear a CROS hearing aid and cannot receive a cochlear implant due to a damaged auditory nerve.
Middle Ear Implants
Middle ear implants also have 2 different applications. It can be implanted on the incus (one of the little bones in the middle ear) or directly on the round window (a membrane that separates the middle ear from the cochlea). The incus approach is indicated for hearing losses that affect the cochlea or the hearing nerve (sensorineural hearing losses) with an intact middle ear. The round window approach is indicated for those with sensorineural or mixed (middle ear and inner ear) hearing losses.
How do we know implantable devices are safe?
It is reassuring to know that hearing implant companies need to follow very strict protocols before an implantable device can become commercially available in Australia. The organization responsible for approval is the Therapeutic Goods Administration (TGA). In principle, a hearing implant is indicated only when a conventional hearing aid is not sufficient to provide adequate hearing. The question is how to define adequate hearing? What is appropriate for some may not be satisfactory for others.
How are the hearing outcomes measured?
Manufacturers provide funding for clinical trials and research to compare results and performance. Comparisons are usually based on the users’ ability to recognize speech with the different devices. Such research can be biased and used for marketing purposes.
Healthy Hearing & Balance Care’s audiologists have a science background which allows them to critically analyze research methods and results to help patients make an informed and unbiased decision.
It is important to remember that as implantable technology progresses, so do conventional hearing aids. In many cases the same end result may be obtained with either conventional or implantable hearing devices depending on the characteristics of each individual.
How are hearing aids and devices funded in Australia?
Implantable hearing devices are fully covered by private health funds as they are part of the surgical prosthesis schedule of fees.
On the other hand, private health funds only reimburse a minimal amount for conventional hearing aids while they are fully subsidized by the government for children up to 26 years of age and over 65s on an age pension.
Who sets the guidelines for device recommendation?
Usually the manufacturers of the device themselves set the criteria of an individual’s suitability for their products. They usually have charts and tables showing the audiogram (hearing test results) of people who would benefit from their hearing devices. Surely no one knows a product better than those who make them. Their candidacy recommendations are usually correct.
There are however several overlaps in the candidacy amongst many of the devices available. It means that a person with a given hearing profile may benefit from several different technology options, either conventional or implantable.
How to Choose the Correct Hearing Aid Device?
Who do you trust?
It is inevitable that each one will preach what they know best.
The manufacturers may tell you that their own products are the best.
The surgeons may tell you that implantable devices are the best.
Audiologists who fit hearing aids only may tell you that hearing aids are the best.
Audiologists who do implants only may tell you that implants are the best.
Consumers who are happy with their own devices will also tell you that theirs are the best.
Implantable and conventional hearing aid technologies are advancing very rapidly. Unfortunately for the implant companies, their devices require much longer clinical trials and more strict approval protocols before their release. For this reason conventional hearing aid technology availability is always ahead of implantable.
This is not to say that conventional hearing aids are any better than implantable technology. There is an important place for implantable devices but candidates must be aware that the selection criteria overlaps. Ideally, before considering surgery, an implant candidate should exhaust all the possibilities of a conventional device.
Another critical point when selecting an implantable device is that the external parts are not interchangeable amongst the different manufacturers. Once you choose one implant you are bound to that company for life.
Manufacturers are constantly perfecting their external processors which are made backwards compatible with the previous generations of their internally implanted parts. This ensures that users are able to update their processors as new technology becomes available.
Who are the candidates for a hearing implant device?
Those with a profound sensorineural hearing loss who have very little or non-recordable hearing as measured through a diagnostic audiometer are straightforward candidates for a cochlear implant, providing they have an intact auditory nerve.
Those with chronic ear discharge, malformed external ears and/or chronic skin allergies which prevent the use of a hearing aid plastic mould in the ear canal, are definitely appropriate candidates for a bone-anchored device providing they have sufficient residual bone conduction hearing thresholds.
What about everybody else in between?
As a rule of thumb, all the others will qualify for more than one technology and may equally benefit from many of them. It is a matter of choosing which is more appropriate for the individual’s overall needs.
What about choosing the correct model?
Some people believe that a device which provides benefit to a friend will automatically be suitable for them. This is usually not the case. To compare the results one needs to consider causes, degree, progression and duration of hearing loss by detailed audiological assessment including lifestyle and communication needs. Even when these factors are identical we may still not obtain the same outcome with the same device due to other individual differences.
Who should make the choice?
Before proceeding with an implant, choose a qualified audiologist that you trust. Have a comprehensive audiological assessment. Explore the latest advances in conventional hearing aid technology. Try the best available for your needs and undergo some auditory training if appropriate. Take advantage of wireless technology such as remote microphones to increase the signal to noise ratio that will improve hearing in noisy environments.
If, after exploring all options, an implant is deemed to be the best choice for you, do not hesitate. Current surgical techniques and implantable technologies are safer than ever.
The following websites will help you to further understand implantable hearing technologies available in Australia:
Contact Form
If you have an enquiry or would like to make an appointment, please call our clinic on (02) 9387 3599. Alternatively, please complete the form below.